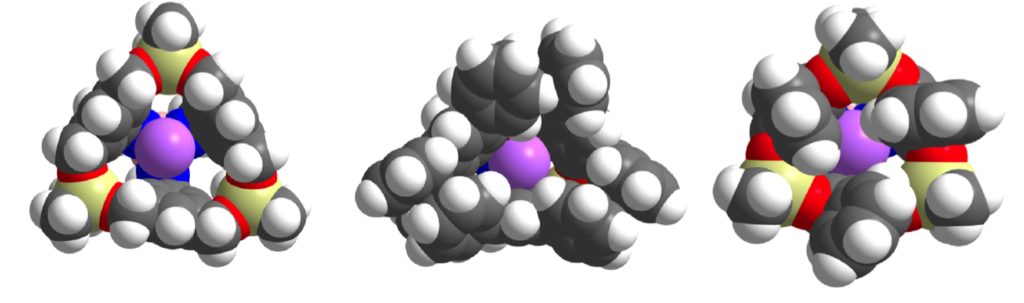
Shape-Selective Catalysis
The pore structures of zeolites and related porous inorganic materials have long been exploited to effect industrially important catalytic reactivity in a shape-selective manner. Here, active sites are accessible via diffusion through rigid channels and apertures, often giving rise to restricted substrate/product transport and, thereby, selectivity. Unfortunately, the tunability of such materials falls far short of that endemic to discreet molecular species which, when exploited as homogeneous catalysts, obviate alternative reactivity at surface and/or defect sites. Accordingly, we are working to develop organometallic species that function as “molecular zeolites”, whereby a metal-based active site is guarded by an effective aperture that dictates substrate diffusion and enforces shape selectivity.
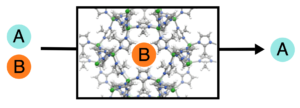
Next-Generation Materials for Molecular Separations
Industrial separations of small molecule mixtures consume staggering quantities of energy, due in large part to their frequent reliance on thermally-driven methods. As potential alternatives, adsorbent and membrane technologies have the potential to afford substantial energy and cost savings. We are interested in the rational design of new types of rigid and soft materials that can display large selectivites yet undergo facile regeneration, and hope to work toward the replacement of established separation processes with lower-cost and greener technologies.
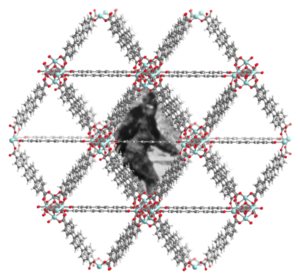
Trapping Elusive Species within Porous Materials
Inert gas matrices have facilitated the spectroscopic characterization of myriad molecular inorganic/organometallic species that have no stable condensed-phase analogues. We are interested exploiting the pores of crystalline framework materials as protective spheres to abet the isolation, characterization, and manipulation of typically fleeting organometallic fragments.

