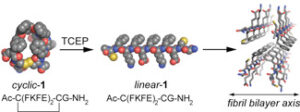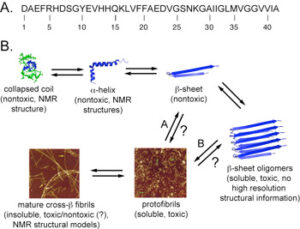
Figure 1: A. Primary amino acid sequence of the Alzheimer’s disease Aβ42 peptide. B. Schematic representation of Aβ self-assembly events.
The precise mechanisms that promote self-assembly of peptides and proteins into amyloid architectures are not well understood. In addition, the relationship between amyloid self-assembly, which proceeds through various transient intermediates en route to amyloid fibrils (Figure 1), and pathology is poorly characterized. We have conducted fundamental studies that clarify how the hydrophobicity, steric profile, and aromatic character of the constituent amino acids of self-assembling peptides influence the formation higher order amyloid structures. These studies form the basis for ongoing efforts in the Nilsson lab that focus on understanding the structure and function of prefibrillar oligomer structures of the Alzheimer’s disease Aβ peptide (Figure 1). Specifically, we are developing ligands that perturb the stability and toxicity of prefibrillar oligomers and we have also identified lead compounds that are cytoprotective against Aβ-mediated toxicity at very low concentrations relative to existing compounds.