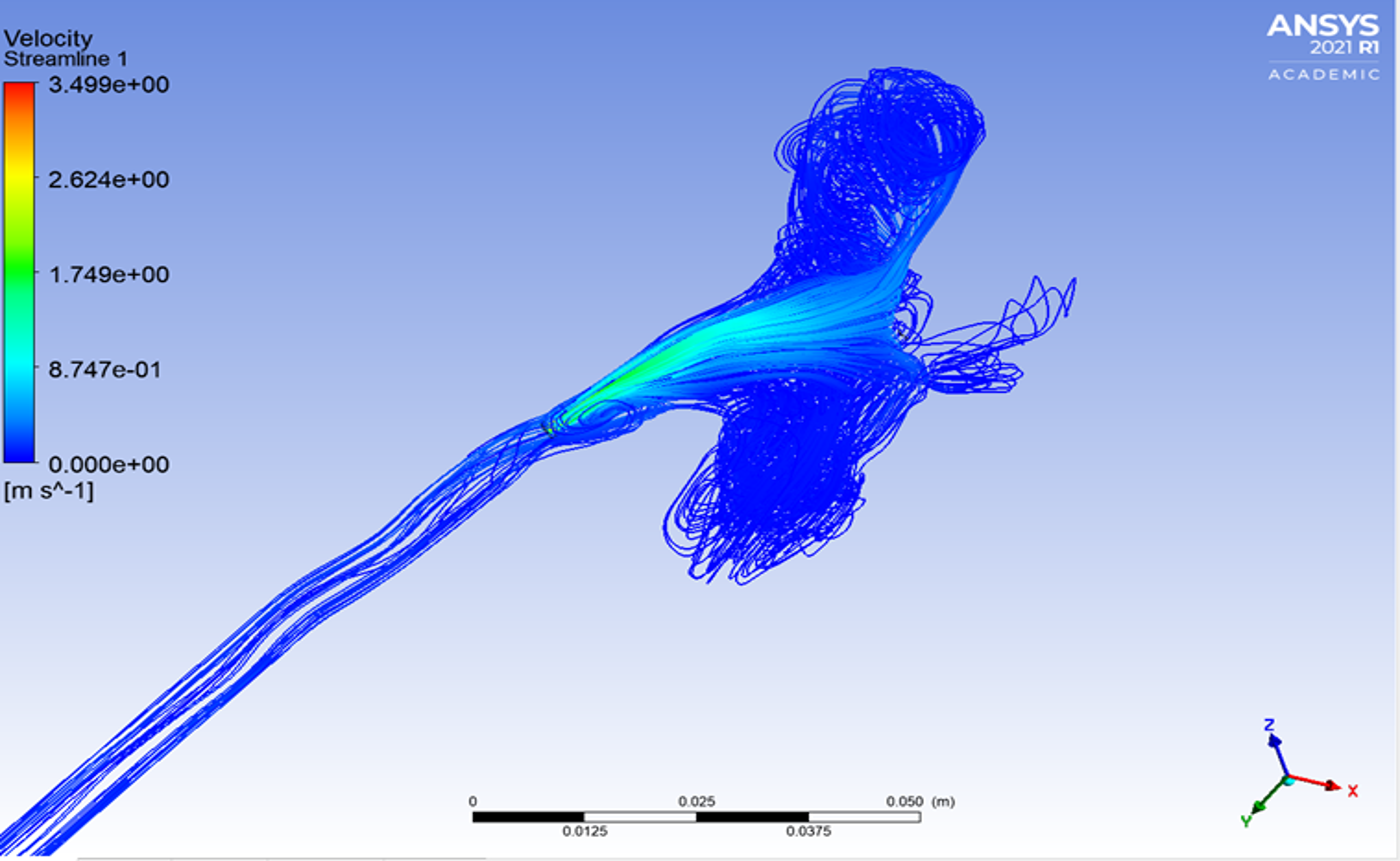
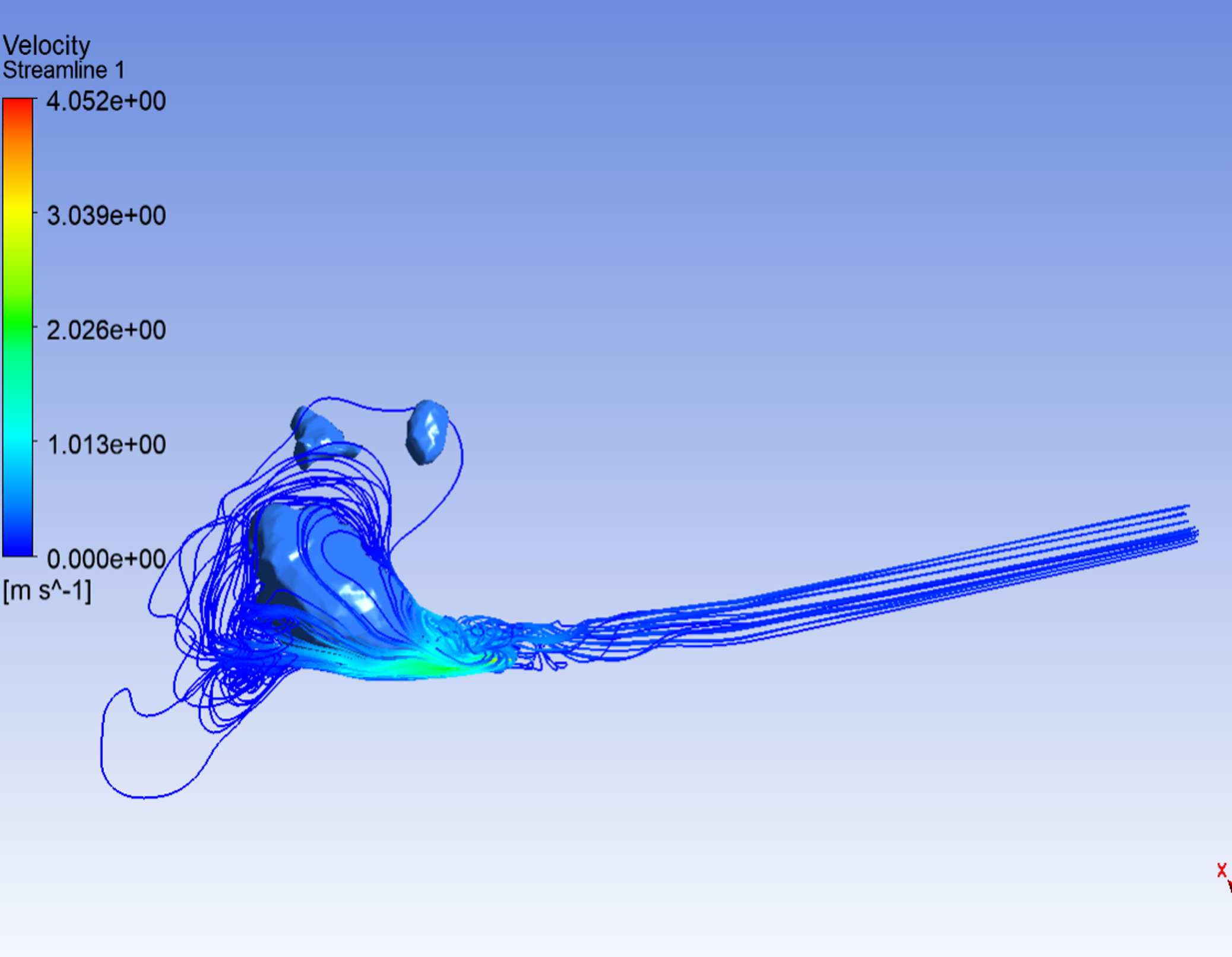
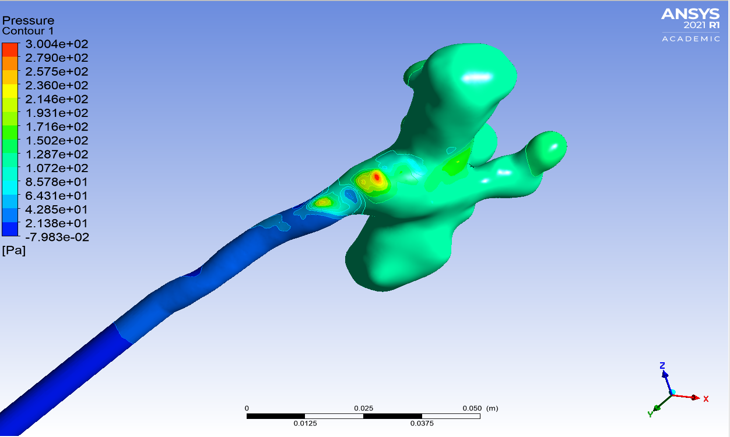
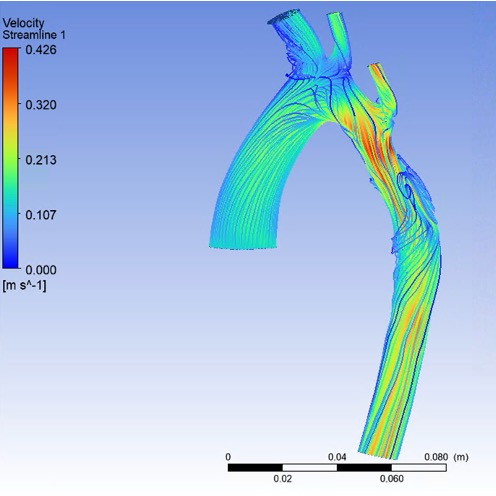
Analysis of Repaired Coarctation of the Aorta Using CFD Simulations
Coarctation of the aorta (CoA) is defined as a stenosis of the descending thoracic aorta (DAo) which results in the narrowing of the aortic arch. Current clinical guidelines recommend intervention when there is significant aortic narrowing, and when a significant pressure gradient is measured during cardiac catheterization. Patients with CoA remain at high risk due to complications including increased mortality, hypertension, stroke, and decreased exercise capacity. This condition effects approximately 6-8% of the population. The aortic arch plays a crucial role in the delivery of blood from the heart to the body. This project aims to model the flow through the aortic arch using computational fluid dynamics (CFD) to analyze the complex flow patterns and pressure gradients in patients pre and post CoA repair with the goal of providing insight to the flow patterns to better understand the effect of the repair, as well as contribute to future repair strategies.
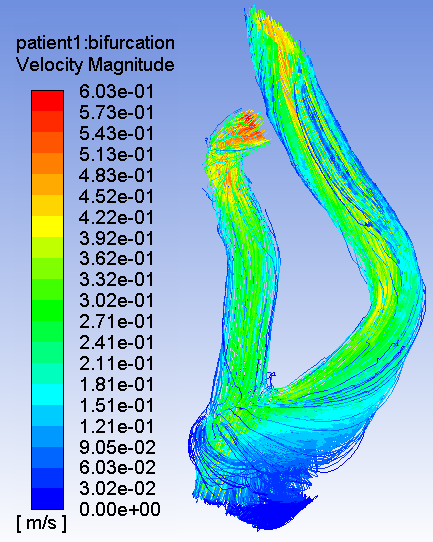
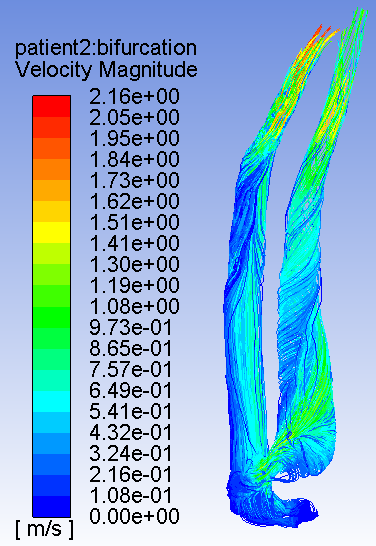
carotid artery blood flow
The purpose of this study is to investigate the potential for Computational Fluid Dynamics (CFD) simulations to predict stroke in patients with carotid artery disease. Using CFD we will study the flow patterns in patient’s carotid artery scans paying specific attention to the geometry, velocity profiles, streamlines and pressure gradients. Using these data, we will computationally simulate each patient’s specific blood flow patterns based on computerized tomography angiography (CTA) for geometry and ultrasound for velocity and pressure data. This study is a feasibility study to understand the differences in CFD simulations of patients that stroked versus patients that did not stroke. These comparisons will be used to understand the potential to predict these actual clinical outcomes using these CFD simulations. If we find that our hypothesis is correct, that CFD can be used to predict the onset of stroke in patients, the study will move on to Phase II where additional patient CTA and ultrasound results will be obtained. Using the learnings of Phase 1, we will determine whether correct predictions for stroke can be determined without knowing the outcome before the CFD analysis is done.
Ureteroscopy Irrigation of the Kidney
This project focuses on modeling the fluids dynamics during ureteroscopy, the primary surgical treatment for kidney stones. The process consists of retrograde passage of a narrowed lighted scope (ureteroscope), under vision through the urinary channel up to the stones. Ureteroscope fluid irrigation through a channel is essential to pressurise the ureter and the kidney to secure a better vision field during lithotripsy and provide a spontaneous flow passage for kidney stone fragments to move out of the kidney.
Computational Fluid Dynamics (CFD) is a branch of mechanics that creates simulations for a variety of applications and is becoming a useful tool in medical applications to study the physiological flow patterns of fluids in the human body. The aim of this project is to utilize CFD to model and monitor the development of the fluid velocity and intrapelvic pressure during ureteroscopy irrigation. Geometries were obtained from scans of patients’ anatomy and inserted into the CFD program. The ureteroscope was placed manually into the geometry at 5 different locations to obtain representative fluid velocity and intrapelvic pressure data that will be validated with known values. Patients’ information and ureteroscope’s operating conditions are provided by the University of Rochester Medical Center so that the simulation has sufficient boundary conditions to replicate an accurate real-life procedure. The flow behavior of the saline solution within the flow domain is governed by the Navier-Stokes equations. Optimization and manipulation of the geometries allow for the creation of an accurate 3-dimensional mesh throughout the geometries where the CFD software uses finite volume methods and iterative procedures to solve the basic governing equations of fluid dynamics and generate several million data points throughout the geometry.
Through the examination of velocity and pressure profiles over time, an extensive guideline can be established to determine the safe operation time for a specific ureteroscope setting. Our objective is to gauge different patients’ specific and unique flow patterns and pressure gradients during the procedure to have a better comprehensive understanding of ureteroscope irrigation.