
James M. Farrar
Professor Emeritus of Chemistry
PhD, University of Chicago, 1974
- Office Location
- B19 Hutchison Hall
- Telephone
- (585) 275-5834
- Web Address
- Website
Research Overview
Professor Farrar and his research group focus their interests on the reaction dynamics and photochemistry of ionic species. The objective of this research is to elucidate the reactivity of gas phase species important in electrical discharges, reactive ion etching, atmospheric and interstellar chemistry, and planetary atmospheres. The research also focuses on understanding processes involved in the transition from the gas phase to the condensed phases, as probed spectroscopically through size-dependent properties of mass-selected cluster ions. The research employs the techniques of mass spectrometry, laser spectroscopy, molecular beams, and computation.
The principal interest of the group is the application of the crossed beam method to studies of low energy ion-molecule reactions. Our research is based on velocity map imaging methods for reconstructing product velocity space distributions by taking a snapshot of the spatial distribution in a particular time window. The locus of points of reaction products with a constant center of mass speed is a sphere whose radius increases with time. Imaging the set of nested spheres describing the reaction products by projecting them on a plane allows all product velocity elements to be observed in a single time window. The schematic diagram in Figure 1 indicates the important elements of the imaging method as implemented in our laboratory.
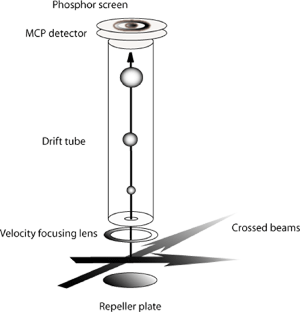
Figure 1.
Our initial studies have been on systems important in ion processing in circumstellar shells and in the atmosphere of Saturn’s moon Titan, which has been probed extensively by the Cassini spacecraft mission. Figure 2 shows product velocity space images for the C+ + NH3 system. The left panel shows the flux resulting from charge transfer; the NH3+ products are formed at the tip of the NH3 velocity vector. The right panel shows the flux arising from the production of HCNH+ via a long-lived complex produced by insertion of C+ into the N-H bond of ammonia.
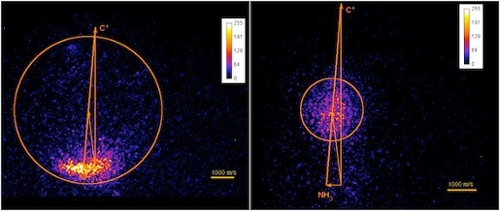
Figure 2.
Our current work is focused on the charge transfer and bond-formation reactions of atomic cations with molecules of importance in atmospheric chemistry, including methyl halides and CFCs. We plan to extend these studies to the study of reactions between ions and simple free radicals. There are virtually no data on such reactions, and we expect that the inclusion of ion-radical chemistry in models for planetary atmospheres, for example, will prove to be essential for a quantitative understanding of chemistry in such environments.
Research Interests
- Dynamical studies of low energy ion-molecule reactions in the gas phase
- imaging studies of collisions
- photochemistry of size-selected ionic clusters
Selected Publications
- Falcinelli, S., Alagia, M., Farrar, J. M., Kalogerakis, K. S., Pirani, F., Richter, R., Schio, L., Stranges, S., Rosi, M., Vecchiocattivi, F. “Angular and Energy Distribution of Fragment Ions in Dissociative Double Photoionization of Acetylene and the 31.9 - 50.0 eV Photon Energy Range”, J. Chem. Phys. 2016, 145, 114308.
- Pei, L., Farrar, J. M. “Velocity Map Imaging Study of Ion-Radical Chemistry: Carbon-Carbon Bond Formation in the Reaction of Allyl Radicals with C+”, J. Phys. Chem. A2016, 120, 6122-6128.
- Pei, L., and Farrar, J. M. “Ion-Molecule Reaction Dynamics: Velocity Map Imaging Studies of N+ and O+ with CD3OD”, J. Chem. Phys. 2015, 143, 084304.
- Pei, L., Carrascosa, E., Yang, N., Falcinelli, S., Farrar, J. M. “A Velocity Map Imaging Study of Charge Transfer and Proton Transfer Reactions of CH3 Radicals with H3+,” J. Phys. Chem. Lett. 2015, 6, 1684-1689.
- Pei, L., Farrar, J. M. “Imaging Ion-Molecule Reactions: Charge Transfer and Halide Transfer Reactions of O+ with CH3Cl, CH3Br, and CH3I,” Int. J. Mass Spectrom. 2015377, 93-100.
- Falcinelli, S., Rosi, M., Candori, P., Vecchiocattivi, F., Farrar, J. M., Pirani, F., Balucani, N., Alagia, M., Richter, R., Stranges, S. "Kinetic Energy Release in Molecular Dication Fragmentation after VUV Ionization and Escape from Planetary Atmospheres," Planetary Space Science 2014, 99, 149-157.
