News Archive
Selective C—H functionalization in complex antimalarial drug via fine-tuned P450 catalysts
 The ability to construct and manipulate biologically active molecules is central to the discovery of compounds with novel or improved pharmacological properties. Methods for the selective functionalization of aliphatic carbon-hydrogen (C—H) bonds are of particularly high synthetic value, as these chemical bonds are ubiquitous in natural and synthetic bioactive molecules. Performing this transformation with high efficiency and selectivity constitutes however a formidable challenge due to the strength of C−H bonds and the presence of several C−H bonds of similar energy in organic compounds, especially in complex molecules. A research team led by Prof. Rudi Fasan has reported a novel strategy to obtain cytochrome P450-based catalysts useful for the late-stage functionalization of unactivated C—H bonds in artemisinin, a complex natural product of prominent value in the fight against malaria (J. Am. Chem. Soc. 2012; 134(45): 18695–704). This impressive work was highlighted in the Chemical & Engineering News and selected for the JACS Spotlight "Enzyme Targets Hard-To-Oxidize Chemical Bonds".
The ability to construct and manipulate biologically active molecules is central to the discovery of compounds with novel or improved pharmacological properties. Methods for the selective functionalization of aliphatic carbon-hydrogen (C—H) bonds are of particularly high synthetic value, as these chemical bonds are ubiquitous in natural and synthetic bioactive molecules. Performing this transformation with high efficiency and selectivity constitutes however a formidable challenge due to the strength of C−H bonds and the presence of several C−H bonds of similar energy in organic compounds, especially in complex molecules. A research team led by Prof. Rudi Fasan has reported a novel strategy to obtain cytochrome P450-based catalysts useful for the late-stage functionalization of unactivated C—H bonds in artemisinin, a complex natural product of prominent value in the fight against malaria (J. Am. Chem. Soc. 2012; 134(45): 18695–704). This impressive work was highlighted in the Chemical & Engineering News and selected for the JACS Spotlight "Enzyme Targets Hard-To-Oxidize Chemical Bonds".
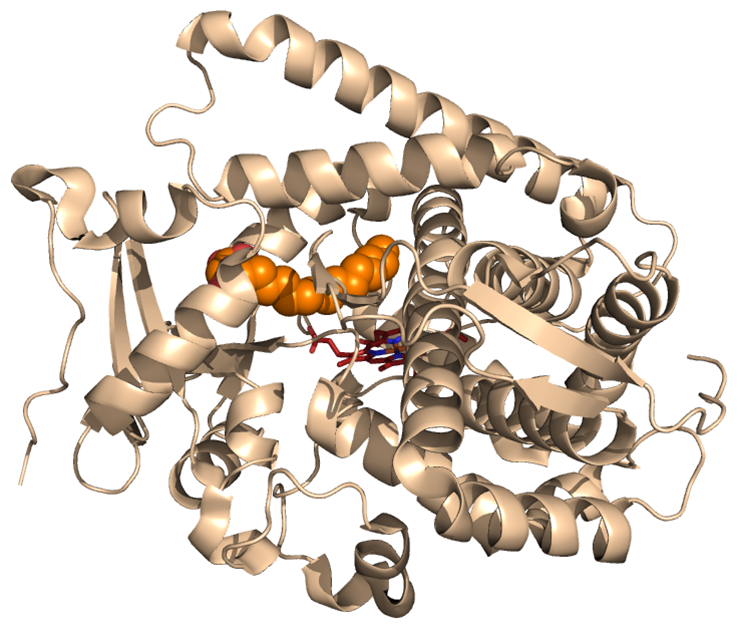
Figure 1: Cytochrome P450 enzyme
Cytochrome P450 monooxygenases are a class of enzymes that utilize molecular oxygen to catalyze the insertion of an oxygen atom into aromatic and aliphatic C—H bonds, among other reactions (Figure 1). While natural P450s can possess exquisite site-selectivity in the oxidation of their native substrates, their activity and selectivity in the oxidation of non-native substrates is typically very poor. Focusing on artemisinin as the target substrate, the Fasan's team has devised a systematic, three-tier strategy that integrates experimental and computational tools to generate and isolate P450 catalysts with tailored site-selectivity for the hydroxylation of 'isolated' secondary and primary C—H bonds in a relevant yet previously inaccessible region of this complex natural product. In a first step, a large library of engineered P450 variants was generated by simultaneous mutagenesis of multiple amino acid residues located within the enzyme active site. Then, the active site shape and geometry of these engineered P450 variants were 'mapped' by means of a high-throughput screen to yield a 'fingerprint' for each member of the library. In the third step, these fingerprints were computationally analyzed to predict and rank those P450 variants that were expected to be most active toward artemisinin hydroxylation and exhibit largest variations in regio/stereoselectivity.
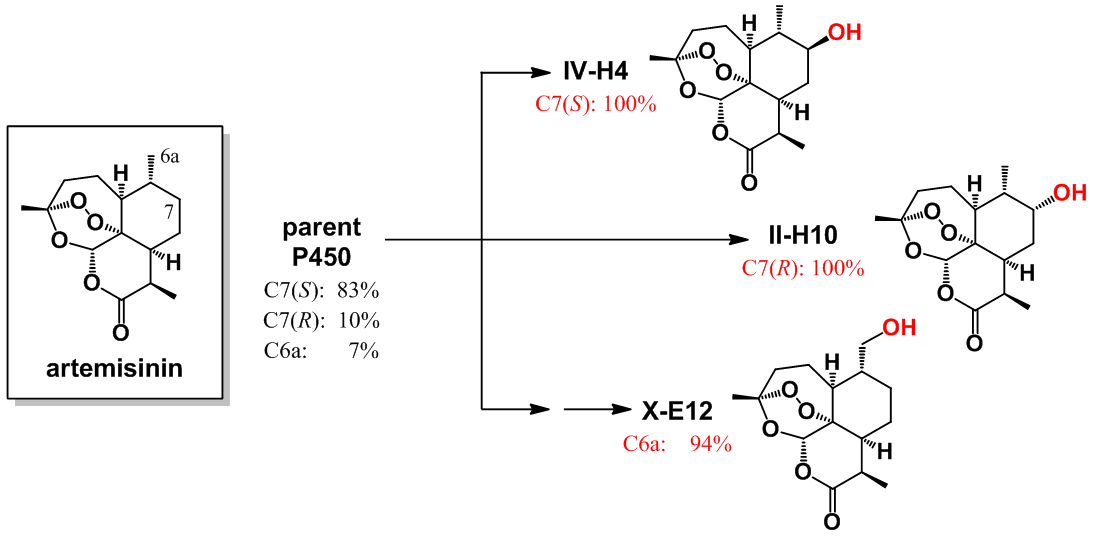
Figure 2: Artemisinin hydroxylation with P450 catalysts with refined selectivity
This procedure enabled the rapid development of three P450 catalysts with excellent regio- and stereoselectivity for the hydroxylation of three distinct aliphatic C—H bonds within a region of the artemisinin skeleton which has remained so far inaccessible to chemical transformation (Figure 2). Whereas achieving this degree of site-selectivity in the P450-mediated oxidation of a non-native substrate remains unprecedented in the field, the entire procedure was also cost- and time-effective, requiring the empirical testing of less than one hundred P450 variants out of the 15,000 present in the original library. Furthermore, since the aliphatic sites targeted by these tailored-made P450 catalysts are involved in the metabolic breakdown of this antimalarial drug within the human body, it becomes possible now to chemically manipulate this molecule in order to obtain derivatives with potentially increased in vivo half-life and potency. As a first step in this direction, the team demonstrated the utility of these biocatalysts for the chemoenzymatic synthesis of enantiopure C7-fluorinated derivatives of the clinically adopted antimalarial drugs artesunate and artemether, in which a major metabolically sensitive site is protected by means of a C−H to C−F substitution (Figure 3).
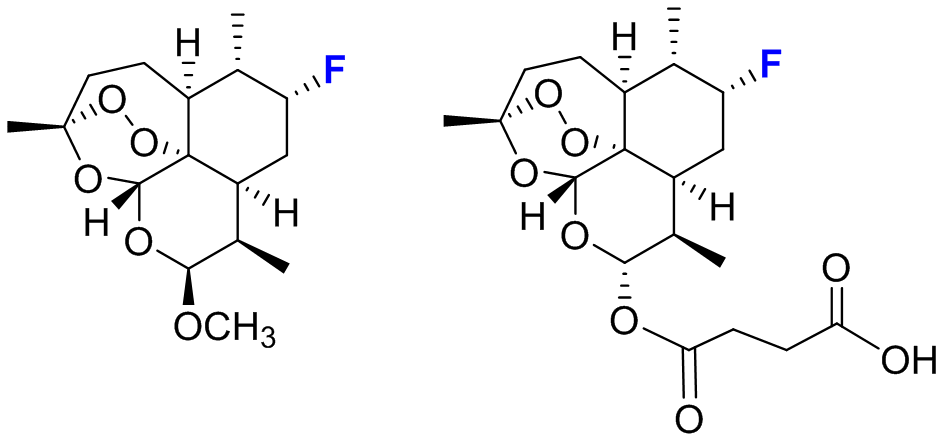
Figure 3: Fluorinated derivatives of antimalarial drugs artemether (left) and artesunate (right)
This work provides a first validation of a rationally-driven strategy for obtaining P450 oxidation catalysts with refined site-selectivity, which could be potentially very general. The Fasan group is currently investigating the scope of this approach in the context of other high-value compounds. Because of the expected impact of these methodologies toward enabling the functionalization of unactivated aliphatic C—H bonds in organic molecules, an international patent application covering this technology has been filed in collaboration with the Office of Technology Transfer of the University of Rochester.
